
Third-Law entropies
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص93-94
الجزء والصفحة:
ص93-94
 2025-11-06
2025-11-06
 32
32
Third-Law entropies
Entropies reported on the basis that S (0) =0 are called Third-Law entropies (and often just ‘entropies’). When the substance is in its standard state at the temperature T, the standard (Third-Law) entropy is denoted So(T). A list of values at 298 K is given in Table 3.3. The standard reaction entropy, ∆rSo, is defined, like the standard reaction en thalpy, as the difference between the molar entropies of the pure, separated products and the pure, separated reactants, all substances being in their standard states at the specified temperature:
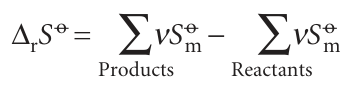
In this expression, each term is weighted by the appropriate stoichiometric coefficient. Standard reaction entropies are likely to be positive if there is a net formation of gas in a reaction, and are likely to be negative if there is a net consumption of gas.
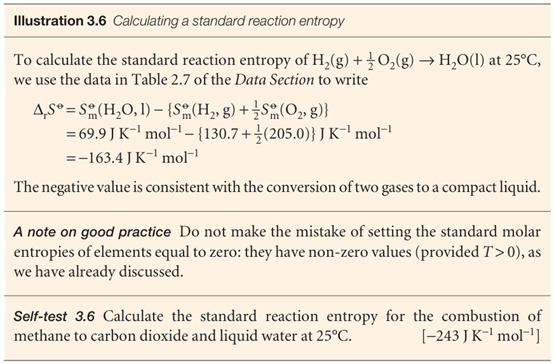
Just as in the discussion of enthalpies in Section 2.8, where we acknowledged that solutions of cations cannot be prepared in the absence of anions, the standard molar entropies of ions in solution are reported on a scale in which the standard entropy of the H+ ions in water is taken as zero at all temperatures:
So (H+, aq) = 0
The values based on this choice are listed in Table 2.7 in the Data section.5 Because the entropies of ions in water are values relative to the hydrogen ion in water, they may be either positive or negative. A positive entropy means that an ion has a higher molar entropy than H+ in water and a negative entropy means that the ion has a lower molar entropy than H+ in water. For instance, the standard molar entropy of Cl−(aq) is +57 J K−1 mol−1 and that of Mg2+(aq) is −128 J K−1 mol−1. Ion entropies vary as expected on the basis that they are related to the degree to which the ions order the water molecules around them in the solution. Small, highly charged ions induce local structure in the surrounding water, and the disorder of the solution is decreased more than in the case of large, singly charged ions. The absolute, Third-Law standard molar entropy of the proton in water can be estimated by proposing a model of the structure it induces, and there is some agreement on the value −21 J K−1 mol−1. The negative value indicates that the proton induces order in the solvent.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


