
Criteria for spontaneity
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص95-96
الجزء والصفحة:
ص95-96
 2025-11-06
2025-11-06
 25
25
Criteria for spontaneity
First, consider heating at constant volume. Then, in the absence of non-expansion work, we can write dqV = dU; consequently
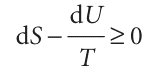
The importance of the inequality in this form is that it expresses the criterion for spontaneous change solely in terms of the state functions of the system. The inequality is easily rearranged to
TdS≥dU (constant V, no additional work)6 At either constant internal energy (dU = 0) or constant entropy (dS = 0), this expression becomes, respectively, dSU,V ≥ 0 dUS,V ≤ 0 , where the subscripts indicate the constant conditions.
Equation 3.26 expresses the criteria for spontaneous change in terms of properties relating to the system. The first inequality states that, in a system at constant volume and constant internal energy (such as an isolated system), the entropy increases in a spontaneous change. That statement is essentially the content of the Second Law. The second inequality is less obvious, for it says that, if the entropy and volume of the system are constant, then the internal energy must decrease in a spontaneous change. Do not interpret this criterion as a tendency of the system to sink to lower energy. It is a disguised statement about entropy, and should be interpreted as implying that, if the entropy of the system is unchanged, then there must be an increase in entropy of the surroundings, which can be achieved only if the energy of the system decreases as energy flows out as heat. When energy is transferred as heat at constant pressure, and there is no work other than expansion work, we can write dqp = dH and obtain
TdS≥dH (constant p, no additional work) , At either constant enthalpy or constant entropy this inequality becomes, respectively, dSH,p ≥ 0 d HS,p ≤ 0 , The interpretations of these inequalities are similar to those of eqn 3.26. The entropy of the system at constant pressure must increase if its enthalpy remains constant (for there can then be no change in entropy of the surroundings). Alternatively, the enthalpy must decrease if the entropy of the system is constant, for then it is essential to have an increase in entropy of the surroundings. Because eqns 3.25 and 3.27 have the forms dU − TdS ≤ 0 and dH − TdS≤0, respectively, they can be expressed more simply by introducing two more thermodynamic quantities. One is the Helmholtz energy, A, which is defined as
A=U−TS The other is the Gibbs energy, G: G=H−TS , All the symbols in these two definitions refer to the system. When the state of the system changes at constant temperature, the two properties change as follows:
(a) dA=dU−TdS (b) dG=dH−TdS , When we introduce eqns 3.25 and 3.27, respectively, we obtain the criteria of spontaneous change as ,(a) dAT,V ≤ 0 (b) dGT,p ≤ 0, These inequalities are the most important conclusions from thermodynamics for chemistry. They are developed in subsequent sections and chapters.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


