
The variation of the Gibbs energy with temperature
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص107-108
الجزء والصفحة:
ص107-108
 2025-11-09
2025-11-09
 50
50
The variation of the Gibbs energy with temperature
As we remarked in the introduction, because the equilibrium composition of a system depends on the Gibbs energy, to discuss the response of the composition to tempera ture we need to know how Gvaries with temperature. The first relation in eqn 3.50, (∂G/∂T) p=−S, is our starting point for this discussion. Although it expresses the variation of Gin terms of the entropy, we can express it in terms of the enthalpy by using the definition of G to write S=(H−G)/T.
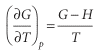
Then We shall see later that the equilibrium constant of a reaction is related to G/T rather than to Gitself,9and it is easy to deduce from the last equation (see the Justification below) that
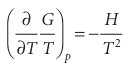
This expression is called the Gibbs–Helmholtz equation. It shows that if we know the enthalpy of the system, then we know how G/T varies with temperature.
Justification 3.5
First, we note that
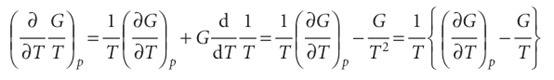
Then we use eqn 3.51 in the form
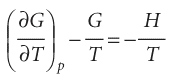
It follows that
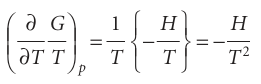
The Gibbs–Helmholtz equation is most useful when it is applied to changes, including changes of physical state and chemical reactions at constant pressure. Then, because ∆G=Gf−Gi for the change of Gibbs energy between the final and initial states and because the equation applies to both Gf and Gi, we can write
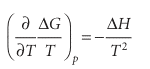
This equation shows that, if we know the change in enthalpy of a system that is undergoing some kind of transformation (such as vaporization or reaction), then we know how the corresponding change in Gibbs energy varies with temperature. As we shall see, this is a crucial piece of information in chemistry.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


