
Concentration Cells and the Glass Electrode
 المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
 المصدر:
College Chemistry
المصدر:
College Chemistry
 الجزء والصفحة:
p 139
الجزء والصفحة:
p 139
 19-7-2017
19-7-2017
 1166
1166
Concentration Cells and the Glass Electrode
Consider a galvanic cell with two Cu electrodes in contact with 0.100 and 0.00100 M solutions of Cu(NO3)2. The standard potential of the cell is E° = 0.000 V. The half-cell and overall cell reactions are:

Such a cell is called a concentration cell since the potential depends only on the concentration ratio for two, otherwise identical, half-cells. This result can be put to good use. Suppose that we want to determine the concentration of a Cu2+ solution. We could place a sample of the solution in contact with a Cu electrode, and join this half-cell with another Cu2+/Cu half-cell with precisely known Cu2+ concentration. The potential of the resulting cell would be a measure of the unknown Cu2+ concentration. The Cu electrode in contact with the Cu2+ solution of unknown concentration is called an indicator electrode, since it ''indicates" the unknown concentration.
An indicator electrode for [Cl-] can be constructed by coating a Ag electrode with insoluble AgCl. The half-cell reaction then is:
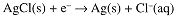
A cell consisting of two Ag/AgCl electrodes in contact with solutions of known and unknown [Cl-] will develop a potential:

The Ag/AgCl electrode is most commonly used as a secondary reference electrode. The hydrogen electrode, the primary reference electrode, is inconvenient to use in practice, since it requires H2(g) and a specially prepared Pt electrode at which the half-reaction is reversible. Thus secondary reference electrodes are usually used in practical electrochemical measurements. A common design for a Ag/AgCl electrode is shown in Figure 15-2. The electrode consists of a Ag wire coated with AgCl, and contained in a tube filled with 0.100 M KCl solution. The tube makes contact with the outer solution through a fiber which allows electrical contact, but with negligible flow of solution. The Ag/AgCl electrode acts as a Cl- indicator electrode because the current which flows to or from such an electrode is carried entirely by Cl- ions. Thus the potential between two Ag/AgCl electrodes is related to the ratio of [Cl-] in contact with the two electrodes. In general, if the electric current is carried by a single species, the potential between solutions A and B is proportional to the logarithm of the concentration ratio CA/CB.
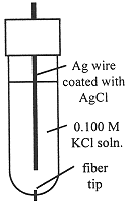
Figure 15-2. A Ag/AgCl electrode.

Figure 15-3. A pH-sensitive glass electrode.
This result has an important application in electrochemical pH measurements (the pH meter) using a glass electrode. A thin membrane of glass can conduct a small electrical current and, in most circumstances, it behaves as though the current carrier is H+. Thus the potential across a glass membrane is proportional to the logarithm of the concentration ratio [H+]inside/[H+]outside. The glass electrode is usually used with another Ag/AgCl/KCl electrode in contact with the test solution. Thus there are three potential-determining processes:
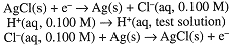
Since both Ag/AgCl electrodes are in contact with the same [Cl-], the potential is determined by the logarithm of the [H+] concentration ratio, and thus is related to the pH of the test solution:
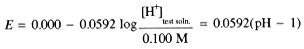
 الاكثر قراءة في الكيمياء الكهربائية
الاكثر قراءة في الكيمياء الكهربائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


