


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-6-2019
Date: 28-8-2019
Date: 15-1-2020
|
The benzyl group (abbv. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. In the case of the benzyl group, it is formed by taking the phenyl group and adding a CH2 group to where the hydrogen was removed. Its molecular fragment can be written as C6H5CH2-R, PhCH2-R, or Bn-R. Nomenclature of benzyl group based compounds are very similar to the phenyl group compounds. For example, a chlorine attached to a benzyl group would simply be called benzyl chloride, whereas an OH group attached to a benzyl group would simply be called benzyl alcohol.
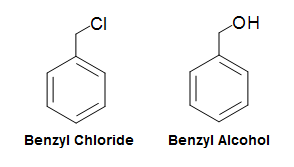
Figure 1.1. Benzyl Group Nomenclature
Additionally, other substituents can attach on the benzene ring in the presence of the benzyl group. An example of this can be seen in the figure below:
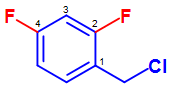
Figure 1.2. Nomenclature of 2,4-difluorobenzyl chloride. Similar to the base name nomenclatures system, the carbon in which th base substitutent is attached on the benzene ring is given the first priority and the rest of the substituents are given the lowest number order possible.
Similar to the base name nomenclature system, the carbon in which the base substituent is attached on the benzene ring is given the first priority and the rest of the substituents are given the lowest number order possible. Under this consideration, the above compound can be named: 2,4-difluorobenzyl chloride.



|
|
|
|
تحذير من "عادة" خلال تنظيف اللسان.. خطيرة على القلب
|
|
|
|
|
|
|
دراسة علمية تحذر من علاقات حب "اصطناعية" ؟!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تحذّر من خطورة الحرب الثقافية والأخلاقية التي تستهدف المجتمع الإسلاميّ
|
|
|