


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 3-1-2022
التاريخ: 2023-08-28
التاريخ: 10-5-2019
التاريخ: 16-9-2019
|
As you saw above, alkenes can be nucleophiles. The reaction of an alkene with HBr is a simple example: the C–C π bond is the HOMO of the nucleophile. The fi rst arrow therefore starts in the middle of the π bond and goes into the gap between one of the carbon atoms and the hydrogen atom of HBr. The second arrow takes the electrons out of the H–Br σ bond and puts them onto the bromine atom to make bromide ion. Overall charge is conserved, so we must generate a positively charged species called a carbocation. The carbocation has a positive charge and an empty p orbital (you can count the electrons to make sure).
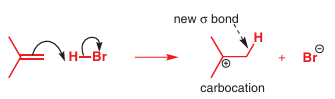
Notice that it was important to draw the two reagents in the right orientation since we need the arrow to show which end of the alkene reacts with which end of HBr. If we had aligned them differently, we would have had trouble drawing the mechanism. Here is a less satisfac-tory representation, in which the H doesn’t seem to transfer to the correct end of the alkene:
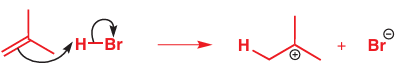
If you fi nd yourself making an ambiguous drawing like this, it is worth having another go to see if you can be clearer. When the nucleophile is a π (or σ) bond rather than a lone pair or a charge there is always the question of which end of the bond actually reacts. One way to make this clear is to draw an atom-specifi c curly arrow actually passing through the atom that reacts. Something like this will do:
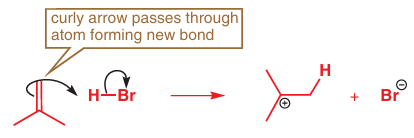
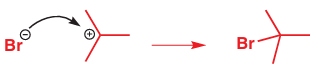
This reaction does not, in fact, stop here as the two ions produced now react with each other to form the product of the reaction. The anion is the nucleophile and the carbocation, with its empty p orbital, is the electrophile.



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
"عراب الذكاء الاصطناعي" يثير القلق برؤيته حول سيطرة التكنولوجيا على البشرية ؟
|
|
|
|
|
|
|
المجمع العلمي يعقد اجتماعاً لمناقشة إطلاق مشروع الدورات القرآنيّة الصيفيّة
|
|
|