

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Halides of the heavy elements
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص385-386
2025-09-08
67
Halides of the heavy elements
Key points: Whereas the halides of nitrogen have limited stability, their heavier congeners form an extensive series of compounds; the trihalides and pentahalides are useful starting materials for the synthesis of derivatives by metathetical replacement of the halide.
The trihalides and pentahalides of Group 15 elements other than nitrogen are used extensively in synthetic chemistry and their simple empirical formulas conceal an interesting and varied structural chemistry. The trihalides range from gases and volatile liquids, such as PF3 (b.p. 102ºC) and AsF3 (b.p. 63ºC), to solids, such as BiF3 (m.p. 649ºC). A common method of preparation is direct reaction of the element and halogen. For phosphorus, the trifluoride is prepared by metathesis of the trichloride and a fluoride:
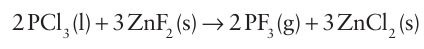
The trichlorides PCl3 , AsCl3 , and SbCl3 are useful starting mate rials for the preparation of a variety of alkyl, aryl, alkoxy, and amino derivatives because they are susceptible to protolysis and metathesis:
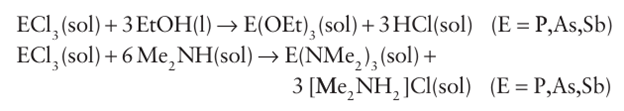
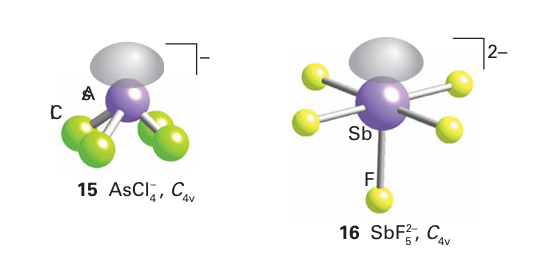
Phosphorus trifluoride, PF3, is an interesting ligand because in some respects it resembles CO. Like CO, it is a weak σ donor but a strong π acceptor, and complexes of PF3 exist that are the analogues of carbonyls, such as Ni (PF3)4, the analogue of Ni (CO)4 (Section 22.18). The π-acceptor character is attributed to a P F antibonding LUMO, which has mainly P p-orbital character. The trihalides also act as mild Lewis acids towards Lewis bases such as trialkylamines and halides. Many halide complexes have been isolated, such as the simple mononuclear species AsCl4 − (15) and SbF5 2− (16). More complex dinuclear and polynuclear anions linked by halide bridges, such as the polymeric chain ([BiBr3 ]2) n in which Bi(I) is surrounded by a distorted octahedron of Br atoms, are also known.
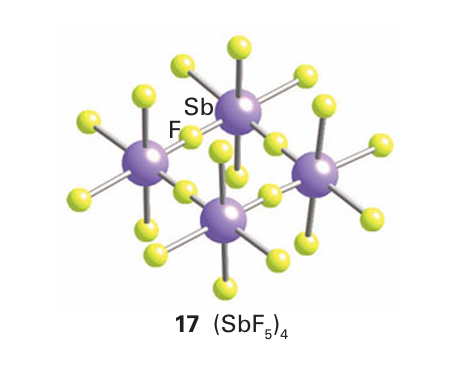
The pentahalides vary from gases, such as PF5 (b.p. 85ºC) and AsF5 (b.p. 53ºC), to solids, such as PCl5 (sublimes at 162ºC) and BiF5 (m.p. 154ºC). The five-coordinate gas-phase molecules are trigonal bipyramidal. In contrast to PF5 and AsF5, SbF5 is a highly viscous liquid in which the molecules are associated through F-atom bridges. In solid SbF5 these bridges result in a cyclic tetramer (17), which reflects the tendency of Sb(V) to achieve a coordination number of 6. A related phenomenon occurs with PCl5, which in the solid state exists as [PCl4][PCl6 −]. In this case, the ionic contribution to the lattice enthalpy provides the driving force for the transfer of a Cl ion from one PCl5 molecule to another. Another contributing factor may be the more efficient packing of the PCl4 and PCl6 units compared to the less efficient stacking of PCl5 units. The pentafluorides of P, As, Sb, and Bi are strong Lewis acids (Section 4.10). SbF5 is a very strong Lewis acid; it is much stronger, for example, than the aluminium halides. When SbF5 or AsF5 is added to anhydrous HF, a superacid is formed. (see Section 4.15):
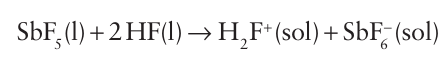
Of the pentachlorides, PCl5 and SbCl5 are stable whereas AsCl5 is very unstable. This difference is a manifestation of the al tarnation effect (Section 9.2c). The instability of AsCl5 is at tributed to the increased effective nuclear charge arising from the poor shielding of the 3d electrons, which leads to a ‘d-block contraction’ and a lowering of the energy of the 4s orbitals in As. Consequently, it is more difficult to promote a 4s electron to form AsCl5.
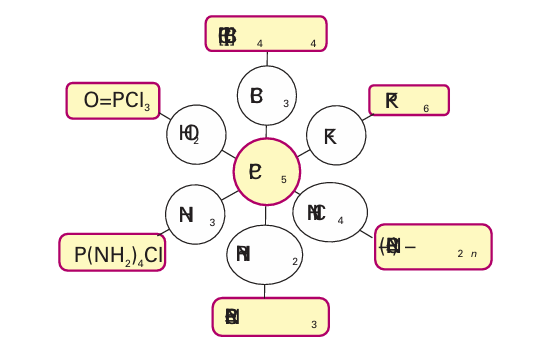
The pentahalides of P and Sb are very useful in syntheses. Phosphorus pentachloride, PCl5, is widely used in the laboratory and in industry as a starting material and some of its characteristic reactions are shown in Fig. 15.5. Note, for example, that reaction of PCl5 with Lewis acids yields PCl4 salts, and simple Lewis bases like F give six-coordinate complexes such as PF6 −. Compounds containing the NH2 group lead to the formation of PN bonds, and the interaction of PCl5 with either H2 O or P4 O10 yields O PCl3.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















