
Space quantization
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
305
الجزء والصفحة:
305
 2025-11-23
2025-11-23
 34
34
Space quantization
The result that ml is confined to the values l, l − 1,..., −l for a given value of l means that the component of angular momentum about the z-axis may take only 2l + 1 values. If the angular momentum is represented by a vector of length proportional to its magnitude (that is, of length {l(l + 1)}1/2 units), then to represent correctly the value of the component of angular momentum, the vector must be oriented so that its projection on the z-axis is of length ml units. In classical terms, this restriction means that the plane of rotation of the particle can take only a discrete range of orientations (Fig. 9.38). The remarkable implication is that the orientation of a rotating body is quantized.
The quantum mechanical result that a rotating body may not take up an arbitrary orientation with respect to some specified axis (for example, an axis defined by the direction of an externally applied electric or magnetic field) is called space quantiza tion. It was confirmed by an experiment first performed by Otto Stern and Walther Gerlach in 1921, who shot a beam of silver atoms through an inhomogeneous mag netic field (Fig. 9.39). The idea behind the experiment was that a rotating, charged body behaves like a magnet and interacts with the applied field. According to classical mechanics, because the orientation of the angular momentum can take any value, the associated magnet can take any orientation. Because the direction in which the mag net is driven by the inhomogeneous field depends on the magnet’s orientation, it fol lows that a broad band of atoms is expected to emerge from the region where the magnetic field acts. According to quantum mechanics, however, because the angular momentum is quantized, the associated magnet lies in a number of discrete orientations, so several sharp bands of atoms are expected. In their first experiment, Stern and Gerlach appeared to confirm the classical pre diction. However, the experiment is difficult because collisions between the atoms in the beam blur the bands. When the experiment was repeated with a beam of very low intensity (so that collisions were less frequent) they observed discrete bands, and so confirmed the quantum prediction.
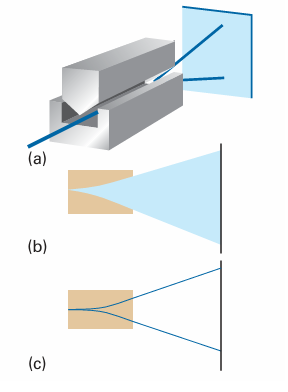
Fig. 9.39 (a) The experimental arrangement for the Stern–Gerlach experiment: the magnet provides an inhomogeneous field. (b) The classically expected result. (c) The observed outcome using silver atoms.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


