

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Compounds of carbon with oxygen and sulfur
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
361-364
2025-09-06
87
Compounds of carbon with oxygen and sulfur
Key points: Carbon monoxide is a key reducing agent in the production of iron and a common ligand in d-metal chemistry; carbon dioxide is much less important as a ligand and is the acid anhydride of carbonic acid; the sulfur compounds CS and CS2 have similar structures to their oxygen analogues. Carbon forms CO, CO2, and the suboxide, O=C=C=C=O (Table 14.3). The uses of CO include the reduction of metal oxides in a blast furnace (Section 5.16) and the shift reaction (Section 10.4) for the production of H2: where we deal with catalysis, we describe the conversion of carbon monoxide to acetic acid and aldehydes. The CO molecule has very low Brønsted basicity and negligible Lewis acidity towards neutral electron-pair donors. Despite its weak Lewis acidity, however, CO is attacked by strong Lewis bases at high pressure and somewhat elevated temperatures. Thus, the reaction with OH- ions yields the formate ion, HCO2-:
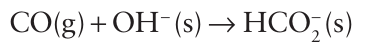
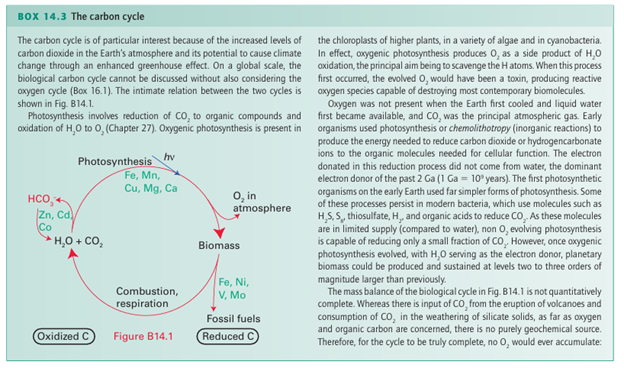
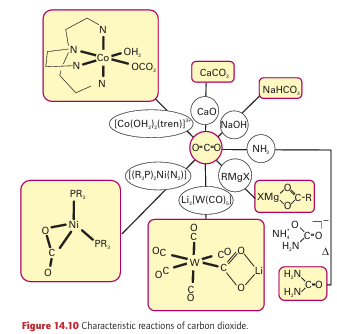
Similarly, the reaction with methoxide ions (CH3 O) yields the acetate ion, CH3CO2. Carbon monoxide is an excellent ligand towards d-metal atoms in low oxidation states (Section 22.5). Its well-known toxicity is an example of this behaviour: it binds to the Fe atom in hemoglobin, so excluding the attachment of O2, and the victim suffocates. An interesting point is that H3BCO can be prepared from B2H6 and CO at high pressures in a rare example of the coordination of CO to a simple Lewis acid. A complex of similar stability is not formed by BF3; this observation is consistent with the classification of BH3 as a soft acid and BF3 as a hard acid. Carbon dioxide is only a very weak Lewis acid. For example, only a small fraction of molecules are complexed with water to form H2CO3 in acidic aqueous solution but, at higher pH, OH coordinates to the C atom, so forming the hydrogencarbonate (bicarbonate) ion, HCO3. This reaction is very slow; yet the attainment of rapid equilibrium between CO2 and HCO3 is so important to life that it is catalysed by a Zn-containing enzyme carbon dioxide hydratase (carbonic anhydrase, Section 27.9a). The enzyme accelerates the reaction by a factor of about 109. Carbon dioxide is one of several polyatomic molecules that are implicated in the greenhouse effect. In this effect, a polyatomic molecule in the atmosphere permits the pas sage of visible light but, because of its vibrational infrared absorptions, it blocks the immediate radiation of heat from the Earth. There is strong evidence for a significant increase in atmospheric CO2 since the industrialization of society. In the past, nature has managed to stabilize the concentration of atmospheric CO2, in part by precipitation of calcium carbon ate in the deep oceans, but it seems that the rate of diffusion of CO2 into the deep waters is too slow to compensate for the increased influx of CO2 into the atmosphere (Box 14.3). There is convincing evidence for increasing concentrations of the greenhouse gases CO2, CH4, N2 O, and chlorofluorocarbon bons, and it is clear that they are having an impact on global temperatures. One method of slowing the rate of increase in atmospheric CO2 is carbon dioxide sequestration (Box 14.4), in which CO2 is captured from industrial flues by reaction with amines. It is then released and liquefied by compression and pumped underground often back into gas or oil wells in order to drive out further oil or gas. From an economic perspective, an important reaction is CO2 with ammonia to yield ammonium carbonate, (NH4)2CO3, which at elevated temperatures is converted directly to urea, CO(NH2)2, a fertilizer, a feed supplement for cattle, and a chemical intermediate. Another important use of CO2 is in the soft drinks industry, where it dissolves under pressure to give a pleasant acidic taste of carbonic acid, H2CO3, and comes out of solution in the form of bubbles when the pressure is released. In organic chemistry, a common synthetic reaction is that be tween CO2 and carbanion reagents to produce carboxylic acids. In the crucial biological process known as the Calvin cycle, CO2 is ‘fixed’ (to the extent of 100 Gt per year) into organic mol ecules by reaction with the electron-rich C=C double bond of a pentose enolate ligand coordinated to a Mg2 ion in the enzyme known as ‘Rubisco’.
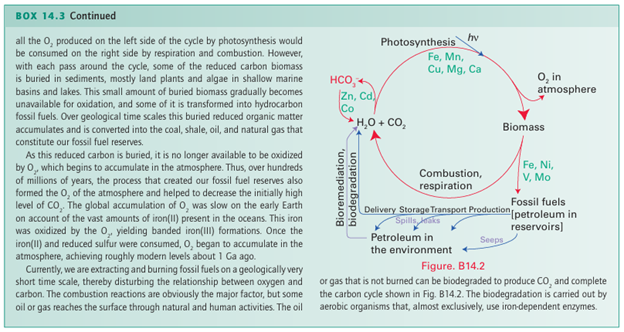
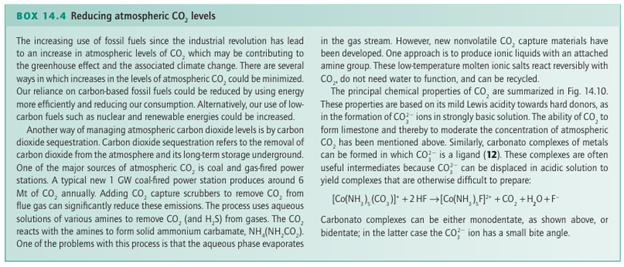
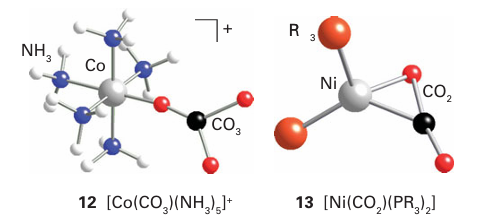
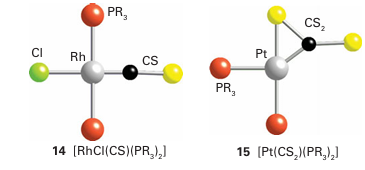
Metal complexes of CO2 are known (13), but they are rare and far less important than the metal carbonyls. In its interaction with a low-oxidation state, electron-rich metal centre, the neutral CO2 molecule acts as a Lewis acid and the bonding is dominated by electron donation from the metal atom into an antibonding π orbital of CO2. An important use of superfluid CO2 (that is, highly com pressed carbon dioxide but above its critical temperature) is as a solvent. Applications range from decaffeination of coffee beans to its use in chemical synthesis in place of conventional solvents as an important part of the strategy for implementing the procedures of ‘green chemistry’. The sulfur analogues of carbon monoxide and carbon dioxide, CS and CS2, are known. The former is an unstable transient molecule and the latter is endoergic (∆fGO 65 kJ mol 1). Some complexes of CS (14) and CS2 (15) exist,
and their structures are similar to those formed by CO and CO2 . In basic aqueous solution, CS2 undergoes hydrolysis and yields a mixture of carbonate ions, CO3-2, and trithio carbonate ions, CS3-2.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















