علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Microporous solids
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص369-371
2025-09-06
88
Microporous solids
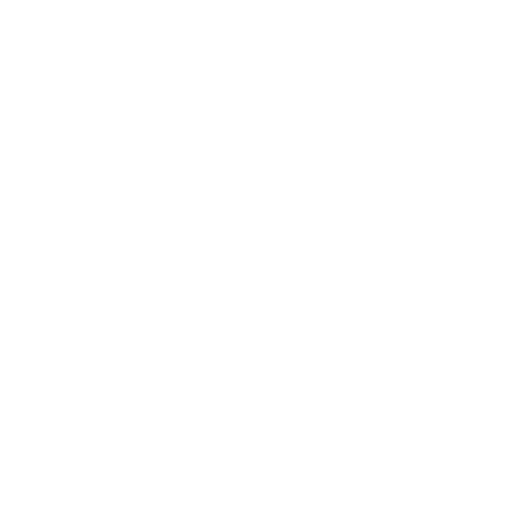
Key point: Zeolite aluminosilicates have large open cavities or channels giving rise to useful properties such as ion exchange and molecular absorption. The molecular sieves are crystalline aluminosilicates having open structures with apertures of molecular dimensions. These ‘micro porous’ substances, which include the zeolites in which cations (typically from Groups 1 or 2) are trapped in an aluminosilicate framework, represent a major triumph of solid-state chemistry, for their synthesis and our understanding of their properties combine challenging determinations of structures, imaginative synthetic chemistry, and important practical applications. The cages are defined by the crystal structure, so they are highly regular and of precise size. Consequently, molecular sieves capture molecules with greater selectivity than high surface area solids such as silica gel or activated carbon, where molecules may be caught in irregular voids between the small particles. Zeolites are used for shape-selective heterogeneous catalysis. For example, the molecular sieve ZSM-5 is used to synthesize 1,2-dimethylbenzene (o-xylene) for use as an octane booster in gasoline. The other xylenes are not produced because the catalytic process is controlled by the size and shape of the zeolite cages and tunnels. This and other applications are summarized in Table 14.6. Synthetic procedures have added to the many naturally occurring zeolite varieties and have produced zeolites that have specific cage sizes and specific chemical properties within the cages. These synthetic zeolites are sometimes made at atmospheric pressure, but more often they are produced in a high-pressure autoclave. Their open structures seem to form around hydrated cations or other large cations such as NR4 ions intro duced into the reaction mixture. For example, a synthesis may be performed by heating colloidal silica to 100 200°C in an autoclave with an aqueous solution of tetra propylammonium hydroxide. The microcrystalline product, which has the typical composition [N (C3H7 )4] OH (SiO2 )48, is converted into the zeolite by burning away the C, H, and N of the quaternary ammonium cation at 500°C in air. Aluminosilicate zeolites are made by in cluding high surface area alumina in the starting materials. A wide range of zeolites has been prepared with varying cage and bottleneck sizes (Table 14.7). Their structures are based on approximately tetrahedral MO4 units, which in the great major ity of cases are SiO4 and AlO4. Because the structures involve many such tetrahedral units, it is common practice to abandon the polyhedral representation in favour of one that emphasizes the position of the Si and Al atoms. In this scheme, the Si or Al atom lies at the intersection of four-line segments and the O atom bridge lies on the line segment (Fig. 14.17). This frame work representation has the advantage of giving a clear impression of the shapes of the cages and channels in the zeolite. Some examples are illustrated in Fig. 14.18. The important zeolites have structures that are based on the ‘sodalite cage’ (Fig 14.3), a truncated octahedron formed by slicing off each vertex of an octahedron (19). The truncation leaves a square face in the place of each vertex and the triangular faces of the octahedron are transformed into regular hexagons. The substance known as ‘zeolite type A’ is based on sodalite cages that are joined by O bridges between the square faces. Eight such sodalite cages are linked in a cubic pattern with a large central cavity called an α cage. The α cages share octagonal faces,
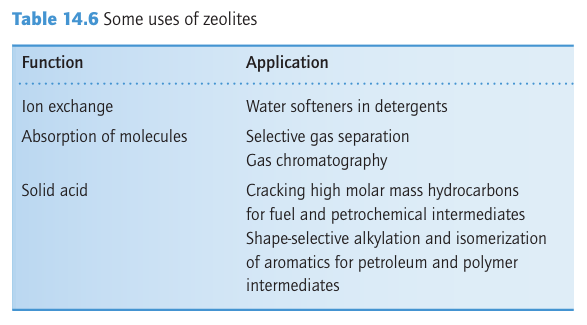
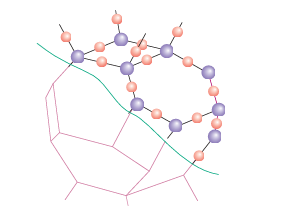
Figure 14.17 Framework representation of a truncated octahedron (truncation perpendicular to the fourfold axes of the octahedron) and the relationship of Si and O atoms to the framework. Note that a Si atom is at each vertex of the truncated octahedron and an O atom is approximately along each edge. with an open diameter of 420 pm. Thus, H2O or other small molecules can fill them and diffuse through octagonal faces. However, these faces are too small to permit the entrance of molecules with van der Waals diameters larger than 420 pm.
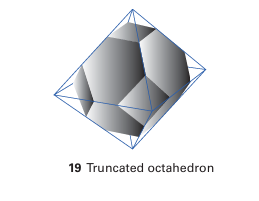
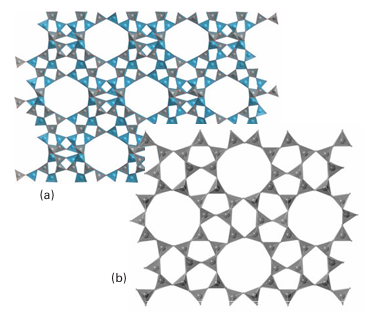
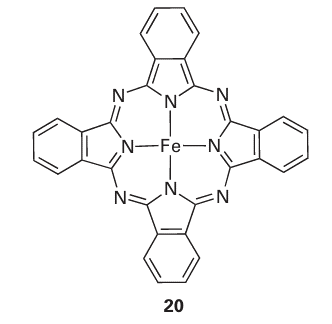
Figure 14.18 Two zeolite framework structures: (a) Zeolite-X and (b) ZSM-5. In each case just the SiO4 tetrahedra that form the framework are shown; nonframework atoms such as charge-balancing cations and water molecules are omitted.A brief illustration. To identify the fourfold and sixfold axes in the truncated octahedral polyhedron used to describe the sodalite cage we note that there is one fourfold axis running through each pair of opposite square faces for a total of three fourfold axes. Similarly, a set of four sixfold axes runs through opposite sixfold faces. The charge on the aluminosilicate zeolite framework is neutralized by cations lying within the cages. In the type-A zeolite, Na ions are present and the formula is Na12 (AlO2 )12 (SiO2)12. xH2O. Numerous other ions, including d-block cations and NH4, can be introduced by ion exchange with aqueous solutions. Zeolites are therefore used for water softening and as a component of laundry detergent to remove the di- and tri-positive ions that decrease the effectiveness of the surfactant. Zeolites have in part replaced polyphosphates because the latter, which are plant nutrients, find their way into natural waters and stimulate the growth of algae. In addition to the control of properties by selecting a zeolite with the appropriate cage and bottleneck size, the zeolite can be chosen for its affinity for polar or nonpolar molecules ac cording to its polarity (Table 14.7). The aluminosilicate zeolites, which always contain charge-compensating ions, have high affinities for polar molecules such as H2O and NH3 . By contrast, the nearly pure silica molecular sieves bear no net electric charge and are nonpolar to the point of being mildly hydrophobic. Another group of hydrophobic zeolites is based on the aluminium phosphate frameworks; AlPO4 is isoelectronic with Si2O4 and the framework is similarly uncharged. One interesting aspect of zeolite chemistry is that large molecules can be synthesized from smaller molecules inside the zeolite cage. The result is like a ship in a bottle because once as sembled the molecule is too big to escape. For example, Na+ ions in a Y-type zeolite may be replaced by Fe+2 ions (by ion exchange). The resulting Fe+2-Y zeolite is heated with phthalonitrile, which diffuses into the zeolite and condenses around the Fe+2 ion to form iron phthalocyanine (20), which remains imprisoned in the cage.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)