

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Aluminosilicates
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص368-369
2025-09-06
93
Aluminosilicates
Key point: Aluminium may replace silicon in a silicate framework to form an aluminosilicate. The brittle layered aluminosilicates are the primary constituents of clay and some common minerals. Even greater structural diversity than that displayed by the silicates themselves is possible when Al atoms replace some of the Si atoms. The resulting aluminosilicates are largely responsible for the rich variety of the mineral world. We have already seen that in γ-alumina, Al3 ions are present in both octahedral and tetrahedral holes (Section 3.3). This versatility carries over into the aluminosilicates, where Al may substitute for Si in tetrahedral sites, enter an octahedral environment external to the silicate framework, or, more rarely, occur with other coordination numbers. Because aluminium occurs as Al (III), its presence in place of Si (IV) in an aluminosilicate renders the overall charge negative by one unit. An additional cation, such as H, Na, or half as many Ca2, is therefore required for each Al atom that replaces an Si atom. As we shall see, these additional cations have a profound effect on the properties of the materials. Many important minerals are varieties of layered aluminosilicates that also contain metals such as Li, Mg, and Fe: they include clays, talc, and various micas. In one class of layered aluminosilicate the repeating unit consists of a silicate layer with the structure shown in Fig. 14.15. An example of a simple aluminosilicate of this type (simple, that is, in the sense of there being no additional
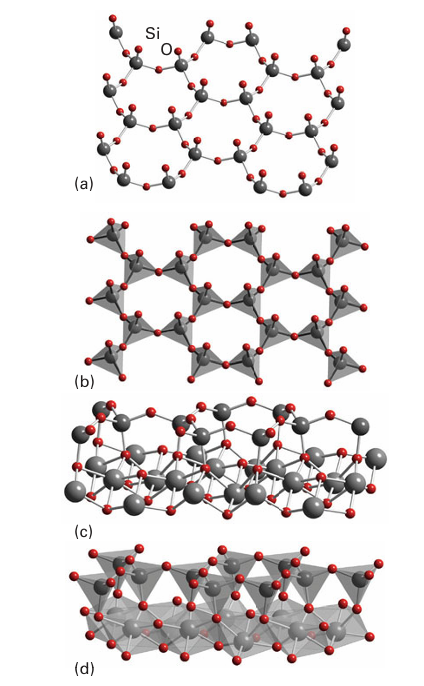
Figure 14.15 (a) A net of SiO4 tetrahedra and (b) its tetrahedral representations. (c) Edge view of the above net and (d) its polyhedral representation. The structures (c) and (d) represent a double layer from the mineral chrysotile, for which M is Mg. When M is Al3+ and the anions in the bottom layers are replaced by an OH- group this structure is close to that of the 1:1 clay mineral kaolinite.
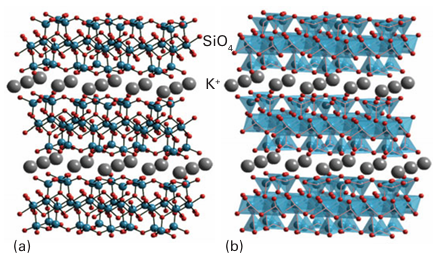
Figure 14.16 (a) The structure of 2:1 clay mineral such as muscovite mica KAl2 (OH)2 Si3 AlO10, in which K resides between the charged layers (exchangeable cation sites), Si4 resides in sites of coordination number 4, and Al+3 in sites of coordination number 6. (b) The polyhedral representation. In talc, Mg2+ ions occupy the octahedral sites and O atoms on the top and bottom are replaced by OH groups and the K+ sites are vacant.elements is the mineral kaolinite, Al2 (OH)4 Si2O5, which is used commercially as china clay. The electrically neutral layers are held together by rather weak hydrogen bonds, so the mineral readily cleaves and incorporates water between the layers. A larger class of aluminosilicates has Al3 ions sandwiched between silicate layers (Fig. 14.16). One such mineral is pyro phyllite, Al2 (OH)2Si4O10. The mineral talc, Mg3 (OH)2 Si4O10 , is obtained when three Mg2 ions replace two Al3 ions in the octahedral sites. As remarked earlier, in talc (and in pyrophyllite) the repeating layers are neutral, and as a result talc readily cleaves between them. Muscovite mica, KAl2(OH)2 Si3 AlO10 , has charged layers because one Al(III) atom substitutes for one Si (IV) atom in the pyrophyllite structure. The resulting negative charge is compensated by a K ion that lies between the repeat ing layers and results in greater hardness. There are many minerals based on a three-dimensional aluminosilicate framework. The feldspars, for instance, which are the most important class of rock-forming minerals (and contribute to granite), belong to this class. The aluminosilicate frameworks of feldspars are built up by sharing all vertices of SiO4 or AlO4 tetrahedra. The cavities in this three-dimensional network accommodations such as K and Ba+2 . Two examples are the feldspars orthoclase, KAlSi3O8 , and albite, NaAlSi3O8.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















