

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Nitrogen activation
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
381
2025-09-07
64
Nitrogen activation
Key points: The commercial Haber process requires high temperatures and pressures to yield ammonia, which is a major ingredient in fertilizers and an important chemical intermediate. Nitrogen occurs in many compounds, but N2 itself, with a triple bond between the two atoms, is strikingly unreactive. A few strong reducing agents can transfer electrons to the N2 molecule at room temperature, leading to scission of the N-N bond, but usually the reaction needs extreme conditions. The prime example of this reaction is the slow reaction of lithium metal at room tempera ture, which yields Li3N. Similarly, when Mg (the diagonal neighbor of Li) burns in air it forms the nitride as well as the oxide. The slowness of the reactions of N2 appears to be the result of several factors. One is the strength of the N-N triple bond and hence the high activation energy required for breaking it. (The strength of this bond also accounts for the lack of nitro gen allotropes.) Another factor is the relatively large size of the HOMO LUMO gap in N2 (Section 2.8b), which makes the molecule resistant to simple electron-transfer redox processes. A third factor is the low polarizability of N2, which does not encourage the formation of the highly polar transition states that are often involved in electrophilic and nucleophilic displacement reactions. Cheap methods of nitrogen activation, its conversion into useful compounds, are highly desirable because they would have a profound effect on the economy, particularly in poorer agricultural economies. In the Haber process for the production of ammonia, H2 and N2 are combined at high tempera tures and pressures over an Fe catalyst, as we discuss in detail in Section 15.10. Much of the recent research aimed at achieving more economical ways of activating N2 has been inspired by the way in which bacteria carry out the transformation at room temperature. Catalytic conversion of nitrogen to NH4 involves the metalloenzyme nitrogenase, which occurs in nitrogen-fixing bacteria such as those found in the root nodules of legumes. The mechanism by which nitrogenase carries out this reaction, at an active site containing Fe, Mo, and S, is the topic of considerable research. In this connection, dinitrogen complexes of metals were discovered in 1965, at about the same time that it was realized that nitrogenase contains Mo (Section 27.13). These developments led to optimism that efficient homogeneous catalysts might be developed in which metal ions would coordinate to N2 and promote its reduction. Many N2 complexes have in fact been prepared, and in some cases the preparation is as simple as bubbling N2 through an aqueous solution of a complex:
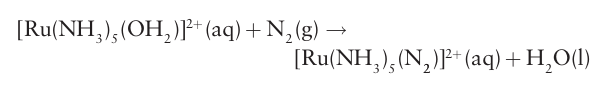
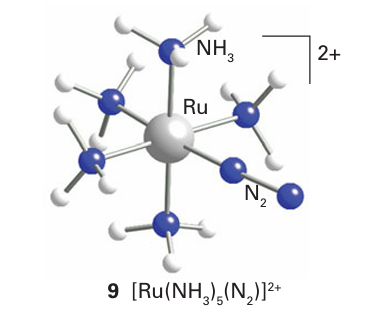
As with the isoelectronic CO molecule, end-on bonding is typical of N2 when it acts as a ligand (9, Section 22.17). The NN bond length in the Ru (II) complex is only slightly altered from that in the free molecule. However, when N2 is coordinated to a more strongly reducing metal centre, this bond is consider ably lengthened by back-donation of electron density into the π* orbitals of N2.
A recent advance has been the direct reduction of N2 to ammonia at room temperature and atmospheric pressure with a molybdenum catalyst that contains a tetradentate triamidoamine ligand known as HIPTN3 N. Nitrogen coordinates to the Mo centre and is converted to NH3 on addition of a proton source and a reducing agent.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















