
Penetration and shielding
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص339-340
الجزء والصفحة:
ص339-340
 2025-11-25
2025-11-25
 43
43
Penetration and shielding
Unlike in hydrogenic atoms, the 2s and 2porbitals (and, in general, all subshells of a given shell) are not degenerate in many-electron atoms. As will be familiar from introductory chemistry, an electron in a many-electron atom experiences a Coulombic repulsion from all the other electrons present. If it is at a distancer from the nucleus, it experiences an average repulsion that can be represented by a point negative charge located at the nucleus and equal in magnitude to the total charge of the electrons within a sphere of radius r(Fig. 10.19). The effect of this point negative charge, when averaged over all the locations of the electron, is to reduce the full charge of the nucleus from Ze to Zeff, the effective nuclear charge. In everyday parlance, Zeff itself is com monly referred to as the ‘effective nuclear charge’. We say that the electron experiences a shielded nuclear charge, and the difference between Z and Zeff is called the shielding constant, σ: Zeff=Z−σ .
The electrons do not actually ‘block’ the full Coulombic attraction of the nucleus: the shielding constant is simply a way of expressing the net outcome of the nuclear attraction and the electronic repulsions in terms of a single equivalent charge at the centre of the atom. The shielding constant is different for s and p electrons because they have different radial distributions (Fig. 10.20). An s electron has a greater penetration through inner shells than a p electron, in the sense that it is more likely to be found close to the nucleus than a p electron of the same shell (the wavefunction of a p orbital, remember, is zero at the nucleus). Because only electrons inside the sphere defined by the loca tion of the electron (in effect, the core electrons) contribute to shielding, an s electron experiences less shielding than a p electron. Consequently, by the combined effects of penetration and shielding, an s electron is more tightly bound than a p electron of the same shell. Similarly, a d electron penetrates less than a p electron of the same shell (recall that the wavefunction of a d orbital varies as r2 close to the nucleus, whereas a p orbital varies as r), and therefore experiences more shielding. Shielding constants for different types of electrons in atoms have been calculated from their wavefunctions obtained by numerical solution of the Schrödinger equation for the atom (Table 10.2). We see that, in general, valence-shell s electrons do experience higher effective nuclear charges than p electrons, although there are some discrepancies. We return to this point shortly. The consequence of penetration and shielding is that the energies of subshells of a shell in a many-electron atom in general lie in the order , s <p<d<f .
The individual orbitals of a given subshell remain degenerate because they all have the same radial characteristics and so experience the same effective nuclear charge. We can now complete the Li story. Because the shell with n = 2 consists of two non-degenerate subshells, with the 2s orbital lower in energy than the three 2p orbitals, the third electron occupies the 2s orbital. This occupation results in the ground-state configuration 1s2 2s1, with the central nucleus surrounded by a complete helium-like shell of two 1s electrons, and around that a more diffuse 2s electron. The electrons in the outermost shell of an atom in its ground state are called the valence electrons because they are largely responsible for the chemical bonds that the atom forms. Thus, the valence electron in Li is a 2s electron and its other two electrons belong to its core.
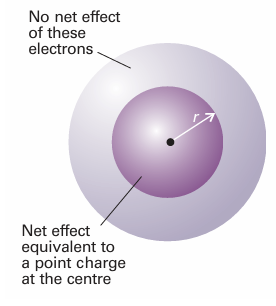
Fig. 10.19An electron at a distance r from the nucleus experiences a Coulombic repulsion from all the electrons within a sphere of radius rand which is equivalent to a point negative charge located on the nucleus. The negative charge reduces the effective nuclear charge of the nucleus from Ze to Zeffe.
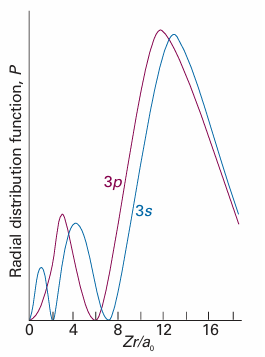
Fig. 10.20 An electron in an s orbital (here a 3s orbital) is more likely to be found close to the nucleus than an electron in a p orbital of the same shell (note the closeness of the innermost peak of the 3s orbital to the nucleus at r = 0). Hence an s electron experiences less shielding and is more tightly bound than a p electron.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


